Purpose: To provide clinicians with evidence-based recommendations for initiation and discontinuation of pharmacologic stress ulcer prophylaxis (SUP)
Section 1: Indication of Pharmacologic Stress Ulcer Prophylaxis Therapy
1. SUP is recommended in the following situations:
a. Patients when mechanical ventilation for more than 48 hours is likely (Strong Recommendation, High Quality of Evidence)1–3
b. Coagulopathy defined as platelet count < 50,000/μL, INR > 1.5, or PTT > 2 times the control value while not on exogenous anticoagulation (Strong Recommendation, High Quality of Evidence)1–4
c. Acute traumatic brain injury with Glasgow Coma Score ≤10 or inability to obey simple commands (Strong Recommendation, Moderate Quality of Evidence)2,3
d. Major thermal injury (≥20% of total body surface area) (Strong Recommendation, Moderate Quality of Evidence)2,3
2. SUP may be considered in the following situations:
a. Acute spinal cord injury (Conditional Recommendation, Low Quality of Evidence)2
b. Partial hepatectomy in the ICU (Conditional Recommendation, Low Quality of Evidence)2
c. Spontaneous subarachnoid hemorrhage, particularly in those with high risk of cerebral vasospasm (or vasospasm present) (Conditional Recommendation, Low Quality of Evidence)5
d. History of GI ulceration or bleeding within 12 months before admission to the ICU (Conditional Recommendation, Very Low Quality of Evidence)2
Section 2: Selection of Pharmacologic Stress Ulcer Prophylaxis Agent:
1. A histamine H2 antagonist (H2RA) or proton pump inhibitor (PPI) are considered first-line agents for SUP (Strong Recommendation, Moderate Quality of Evidence)2,3,6
a. Either a H2RA or PPI may be given first-line for SUP and clinicians should use patient specific factors to guide agent selection (Conditional Recommendation, Very Low Quality of Evidence)
2. Sucralfate is an alternative agent for adults unable to tolerate an H2RA or PPI (Moderate Recommendation, High Quality of Evidence)2
3. An oral or enteral route is preferred for both PPIs and H2RAs when feasible. (Strong Recommendation, Low Quality of Evidence)
Section 3: Discontinuation of Pharmacologic Stress Ulcer Prophylaxis Therapy:
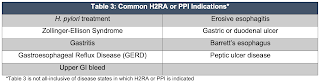
1. SUP should be discontinued if a patient no longer meets an indication listed in Section 1 and does not have an alternate indication (examples included in Table 3) (Strong Recommendation, Moderate Quality of Evidence)
a. Clinicians should assess the indication for stress ulcer prophylaxis daily, upon admission and discharge, and upon a change in the level of care (Strong Recommendation, Moderate Quality of Evidence)11
b. Discontinuation of unnecessary therapy is warranted as acid-suppressive agents have been associated with an increased risk of pneumonia, Clostridium difficile, drug-drug interactions, nutritional deficiencies, and increased costs (Strong Recommendation, Moderate to High Quality of Evidence)12-16
2. SUP may not provide added benefit in patients tolerating enteral nutrition defined as >50% of goal feeds due to improved splanchnic blood flow and possible reduction in gastrointestinal bleeding rates (Conditional Recommendation, Low Quality of Evidence)17–20
a. Literature is unclear regarding the location of feeds (gastric versus post-pyloric) and the effects of reducing the risk for ulceration
b. It may be reasonable to continue SUP therapy despite enteral feeding in patients exhibiting hypersecretory states (i.e. burns, traumatic brain injury)
c. Clinicians are encouraged to individually weigh the risk-benefit assessment when deciding whether to continue or withhold SUP for patients tolerating enteral feeds
3. Considerations should be made for additional indications for H2RA or PPI therapy based on based medical history or current conditions